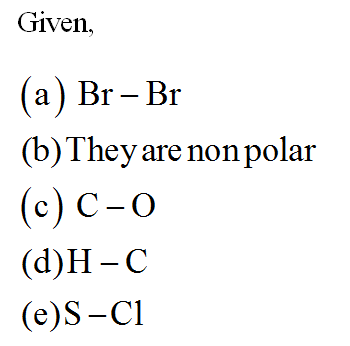CO32- Lewis structure, molecular geometry, bond angle, formal charge, hybridization | Molecular geometry, Molecular, Electron configuration

u ule mass of one mole of electrons. JH 40 respectively. Arrange the following is increasing order of property given () O,F,S, CI, N strength of H-bonding (X-H-X). Secil (ii) N2, 02,

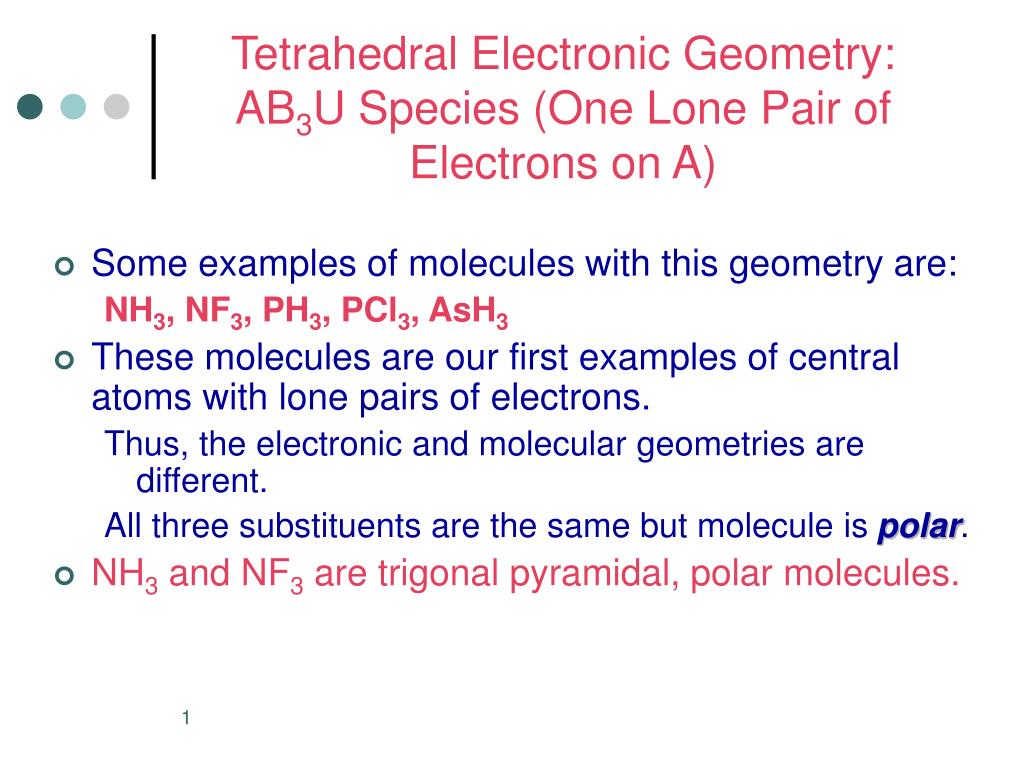
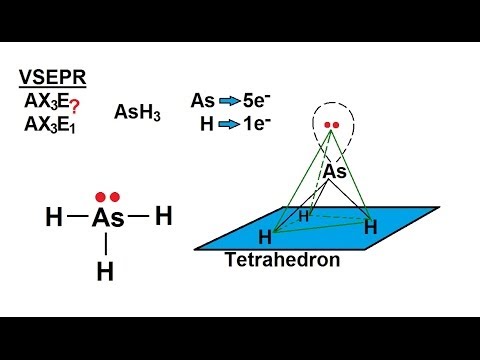
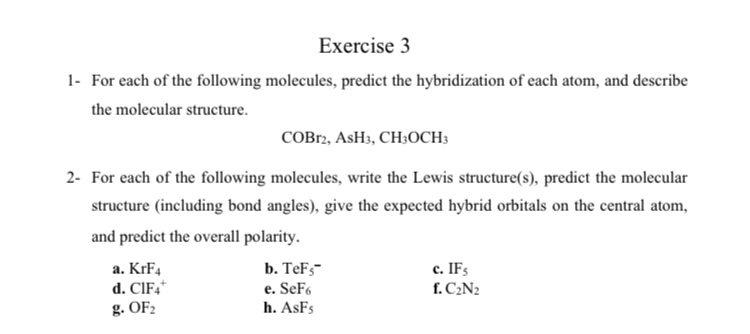
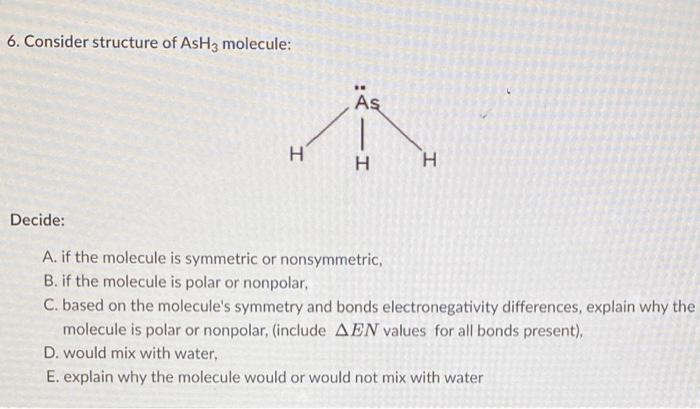
SOLVED: AsH3 vs ClF3. what are the Lewis structure and resonance? Why? What are the electronic geometry? Shape? Bond angles? Polar or nonpolar? why? Hybridization?
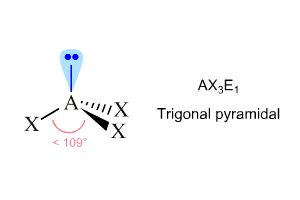
What is the molecular geometry of AsCl3 ? A) tetrahedral B) trigonal pyramidal C) trigonal planar D) T-shaped | Socratic

P Calculate the mass of one mole of electrons. Arrange the following is increasing order of property given (i) O, F,S, CI, N strength of H-bonding (X-H-X). Sa (ii) N2, O2, F2,